Lost: osmosis stuff
 ost readers will know, the great North American series Lost deals with the adventures and misfortunes of some plane crash survivors fighting for their lives as modern Robinson Crusoes in a remote tropical island. When water stored in the plane starts becoming scarce, the idea of drinking water from the ocean comes to Walt’s, the kid in the story’s, mind. His father forbids him to do it, but when the boy asks why he doesn’t know how to answer. He might as well tell him sea water can’t be drunk because it makes you even more thirsty, taking the risk the boy wouldn’t believe it and would try to check it on his own. The wisest thing to do then is to tell him that it is the osmosis’ fault: the boy will be speechless then.
ost readers will know, the great North American series Lost deals with the adventures and misfortunes of some plane crash survivors fighting for their lives as modern Robinson Crusoes in a remote tropical island. When water stored in the plane starts becoming scarce, the idea of drinking water from the ocean comes to Walt’s, the kid in the story’s, mind. His father forbids him to do it, but when the boy asks why he doesn’t know how to answer. He might as well tell him sea water can’t be drunk because it makes you even more thirsty, taking the risk the boy wouldn’t believe it and would try to check it on his own. The wisest thing to do then is to tell him that it is the osmosis’ fault: the boy will be speechless then. Well, the osmosis in question consists of the following: we have two solutions, one of them full of salty water and the other one full of fresh water with little salt. If we link them through a small tube, the less salty solution will give part of his water to the saltier one. This water movement will continue until the concentration of salt is equal in both solutions.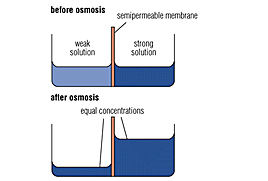
When we drink fresh water, it is absorbed into our bodies by osmosis in an attempt to lower our salt concentration, since our digestive system is saltier than water. But if water is saltier than our body we can’t filter it. Our body will lose water instead, as osmosis will try to reduce sea water’s salt concentrarion (the water movement always goes from the least salted to the more salted area). We will dehydratate even quicker then, and we will hurt our kidneys with salts we can’t filtrate besides.
Osmosis is the w ay plants get water from the ground as well. Plants dry out in very salty soil because they lose water instead of absorbing it, so that their salt concentration becomes the same as that of the soil. That’s why in the past the victor of a war would salt the enemy’s land to destroy the harvest. It is also the reason why river fish can’t live in the sea and vice versa. If we throw a river fish into the sea it will lose water and become reduced and skinny. If we throw a sea fish into the river instead, since it is used to salty water, it will absorb fresh water until it bursts in its attempt to make water concentrations equal inside and outside of its body. Changing from fresh to sea water is traumatic: species that can make it must go through a long and slow adaptation. Osmosis stuff.
ay plants get water from the ground as well. Plants dry out in very salty soil because they lose water instead of absorbing it, so that their salt concentration becomes the same as that of the soil. That’s why in the past the victor of a war would salt the enemy’s land to destroy the harvest. It is also the reason why river fish can’t live in the sea and vice versa. If we throw a river fish into the sea it will lose water and become reduced and skinny. If we throw a sea fish into the river instead, since it is used to salty water, it will absorb fresh water until it bursts in its attempt to make water concentrations equal inside and outside of its body. Changing from fresh to sea water is traumatic: species that can make it must go through a long and slow adaptation. Osmosis stuff.

0 Comments:
Post a Comment
<< Home